Which Statement Describes a Chemical Reaction at Equilibrium
2 Which will not change the rate of a reaction. Reactants and products are consumed and formed at the same rate.

Which Statement Describes A Reaction At Equilibrium Brainly In
All of the products are consumed.

. Which statement best describes a chemical reaction. Which statement describes a chemical reaction at equilibrium. Up to 24 cash back Test 11.
All of the reactants are consumed. It always involves the production of new substances. Which statement correctly describes a chemical reaction at equilibrium.
The forward reaction happens at the same rate as the reverse reaction. The stress can be in any form. Reactants and products are consumed and formed at the same rate.
Terms in this set 25 The net energy released or absorbed during a reversible chemical reaction is equal to. N2g 3H2g -. 1 The concentrations of the products and reactants are equal.
Which statement concerning a chemical reaction at equilibrium is not correct. 2 The concentrations of the products and reactants are constant. There are factors that can disturb this equilibrium and we call them STRESS.
The concentrations of reactants and products are equimolar. Which of the following statement describes a reversible chemical reaction that has reached equilibrium. Chemical equilibrium may be defined as the equilibrium in which the reactants and products.
This is because at equilibrium the rate of the forward reaction equals that of the reverse reaction so even though the reaction doesnt stop there is no net change in the reaction. The reaction is exothermic c. The system is closed and all reactants and products are present.
The value of delta n for a given system is determined by. 3 The rate of the forward reaction is less than the rate of the reverse reaction. Which statement correctly describes a chemical reaction at equilibrium.
Which of the following statements describe a reaction that is at equilibrium. The rate of the forward reaction is less than the rate of the are equal. It always forms a precipitate.
No changes will be apparent as the forward in reverse reactions continue. Equilibrium can be approached from both directions. 4 The rate of the forward reaction is greater than the rate of the reverse reaction.
Which statement correctly describes a chemical reaction at equilibrium. 2 The concentrations of the products and reactants are constant. 1 The concentrations of the products and reactants are equal.
Which statement describes a chemical reaction at equilibrium. The concentrations of the products and reactants D. The forward reaction happens slightly faster than the reverse reaction.
2 The concentrations of the products and reactants are constant. The relationship between Kp and Kc for an equilibrium system is given as KpKc RTdelta n. The reaction has stopped because all reactants have been used up.
A chemical equilibrium is a state of balance in a reaction where the forward and reverse reaction speed is equal and the concentrations of the products and reactants remain unchanged. The chemical reaction above is at equilibrium. Change in concentration of any reactant or product adding or subtracting change in temperature pressure adding a catalyst or change in volume.
Which statement correctly describes a chemical reaction at equilibrium. Which statement describes a chemical reaction at equilibrium. 4 moles of reactant produce 2 moles of product.
That is the rate of forward reaction is equal to the rate of backward reaction. 2 The reactants are completely consumed in the reaction. It always involves the production of gas.
The reaction has stopped until a stress is applied to the system. The concentrations of reactants and products are equimolar. 3 The rate of the forward reaction is less than the rate of the reverse reaction.
Which of the following statements correctly describes the equilibrium state of a chemical reaction. Molecules of reactants and products collide with the same frequency. Reactant molecules and product molecules have the same velocity.
4 The concentrations of the products and reactants are constant. Which statement describes a chemical reaction at equilibrium. Two substances are dissolved in water and start to react which statement accurately describes the appearance of the solution when it reaches equilibrium.
Reactants will convert to products The Gibbs free energy has reached a minimum The forward reaction rate is not equal to the reverse reaction rate O The concentration of product is equal to the concentration of. The partial pressures of the reactants and products. 3 The rate of the forward reaction is.
1 The products are completely consumed in the reaction. The concentrations of the products and reactants C. Which statement correctly describes the equilibrium state of the system There will be more products than reactants at equilibrium COg and Cl2g are combined in a sealed container at 75C and react according to the balanced equation COgCl2g--COCl2g.
The difference between the potential energy of the products and the potential energy of the reactants. The value of Kp for a given reaction is the equilibrium constant based on. Molecules of reactants and products collide with the same frequency.
1 The concentrations of the products and reactants are equal. Chemistry questions and answers. Reactant molecules and product molecules have the same velocity.
There are no changes taking place within the reaction. 3 The concentrations of the products and reactants are equal. The appearance Will continue to change as the forward reaction continues at a slow balanced rate.
The forward and reverse reactions are proceeding at the same rate. CThe reactants change their physical states. The rate of the forward reaction equals the rate of the reverse reaction.
The correct statement is at dynamic equilibrium the reaction continues but the amounts of reactants and products does not change. Equilibrium is the starting point of a chemical reaction or the point at which only reactants are. The concentrations of reactants and products remain constant.
Given the reaction at equilibrium. For example Thus we can conclude that out of the given options statement the concentrations of the products and reactants are equal describes a chemical reaction at equilibrium.
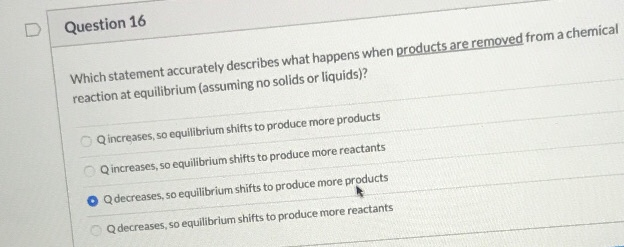
Solved Question 16 Which Statement Accurately Describes What Chegg Com

Welcome To Learnapchemistry Com Ap Chemistry Question Paper Ap Chem

Solved 12 The Value Of Keq Is Temperature Dependent True False 13 Which Of The Following Statements Best Describes Chemical Equilibrium Reactants Form Products As Fast As Products Form Reactants The Concentrations
Comments
Post a Comment